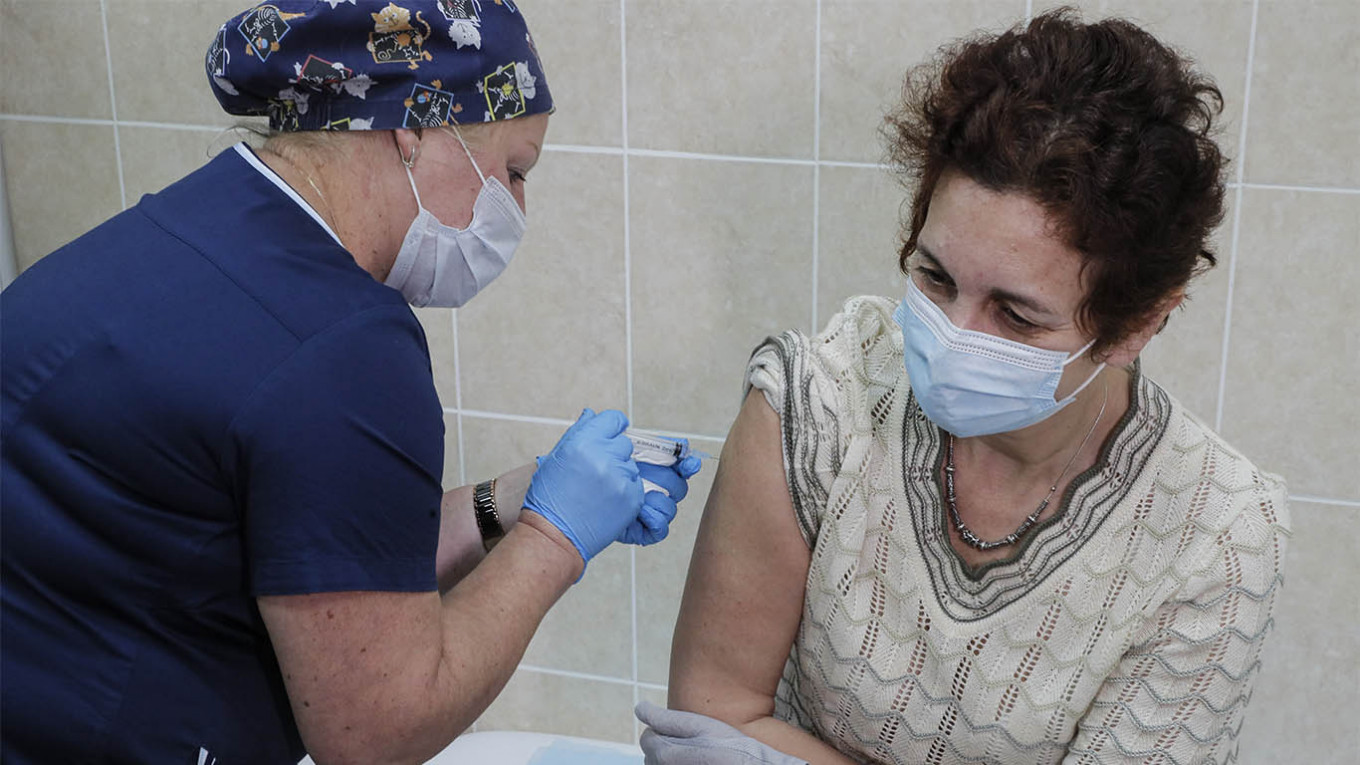
Recruits in Moscow have reported no side effects after taking China’s candidate coronavirus vaccine as part of large-scale clinical trials, the Russian pharmaceutical company working with the vaccine’s Chinese developers announced Monday.
Russia approved Phase 3 trials of the Chinese vaccine developed by CanSino Biologics and the Chinese military’s research arm last month. Russia’s Petrovax pharmaceutical company said it has received more than 3,000 applications to get the Ad5-nCoV vaccine so far.
“At the moment, the volunteers are doing well. None of them have shown any side effects,” Petrovax said in a press release.
The vaccinated subjects are expected to develop an antibody and cellular immune response to Covid-19, the company added.
The study participants will be under direct supervision for nearly a month, with four interim face-to-face examinations, and will undergo a control examination after six months, Petrovax said.
The Russian company said it expects preliminary results sometime in November, according to Interfax.
Once Russia registers the Chinese vaccine, Petrovax said it will be able to produce more than 4 million doses per month this year and 10 million doses per month in 2021.
CanSino announced plans last month to launch Phase 3 trials of the vaccine in Saudi Arabia involving about 5,000 subjects.
Phases 1 and 2 involved more than 700 volunteers in China this spring.
Ad5-nCoV is one of dozens of potential Covid-19 vaccines in various stages of development around the world.
Russia registered its own candidate coronavirus vaccine, Sputnik V, last month before launching Phase 3 trials involving 40,000 volunteers to determine its long-term safety and effectiveness.
