Russia’s Sputnik V coronavirus vaccine is 91.4% effective, its developers announced Monday, citing a new analysis of data from its Phase 3 clinical trials.
The state-run Gamaleya National Center of Epidemiology and Microbiology in Moscow that developed Sputnik V said the data was obtained 21 days after volunteers received the first dose of the two-shot vaccine. The latest 91.4% figure is lower than its previously reported effectiveness of 96.2%.
“The vaccine demonstrated 100% efficacy against severe coronavirus cases. There were 20 severe cases of coronavirus infection among confirmed cases in the placebo group and no severe cases in the vaccine group,” Gamaleya said in a press release.
Data obtained at the third and final control point will be published in the near future in a “leading international peer-reviewed medical journal,” Gamaleya said.
The statement added that developers will use this data to seek accelerated registration for the vaccine in other countries.
The Russian-developed Sputnik V has drawn skepticism at home and abroad after it was rushed through government approval before the start of its large-scale Phase 3 trials. In September, scientists from several countries signed a note of concern over possible data manipulation and statistical anomalies in its Phase 1/2 data published in medical journal The Lancet. Its developers defended their research, saying that the methods they used to obtain measurements give only rough values, not exact numbers.
Phase 3 trials of the adenovirus vector-based vaccine will involve 40,000 volunteers in Moscow. About 26,000 volunteers have already been vaccinated in Russia for the Phase 3 trials with “no unforeseen adverse effects documented,” the Russian Direct Investment Fund that is funding Sputnik V’s development reported.
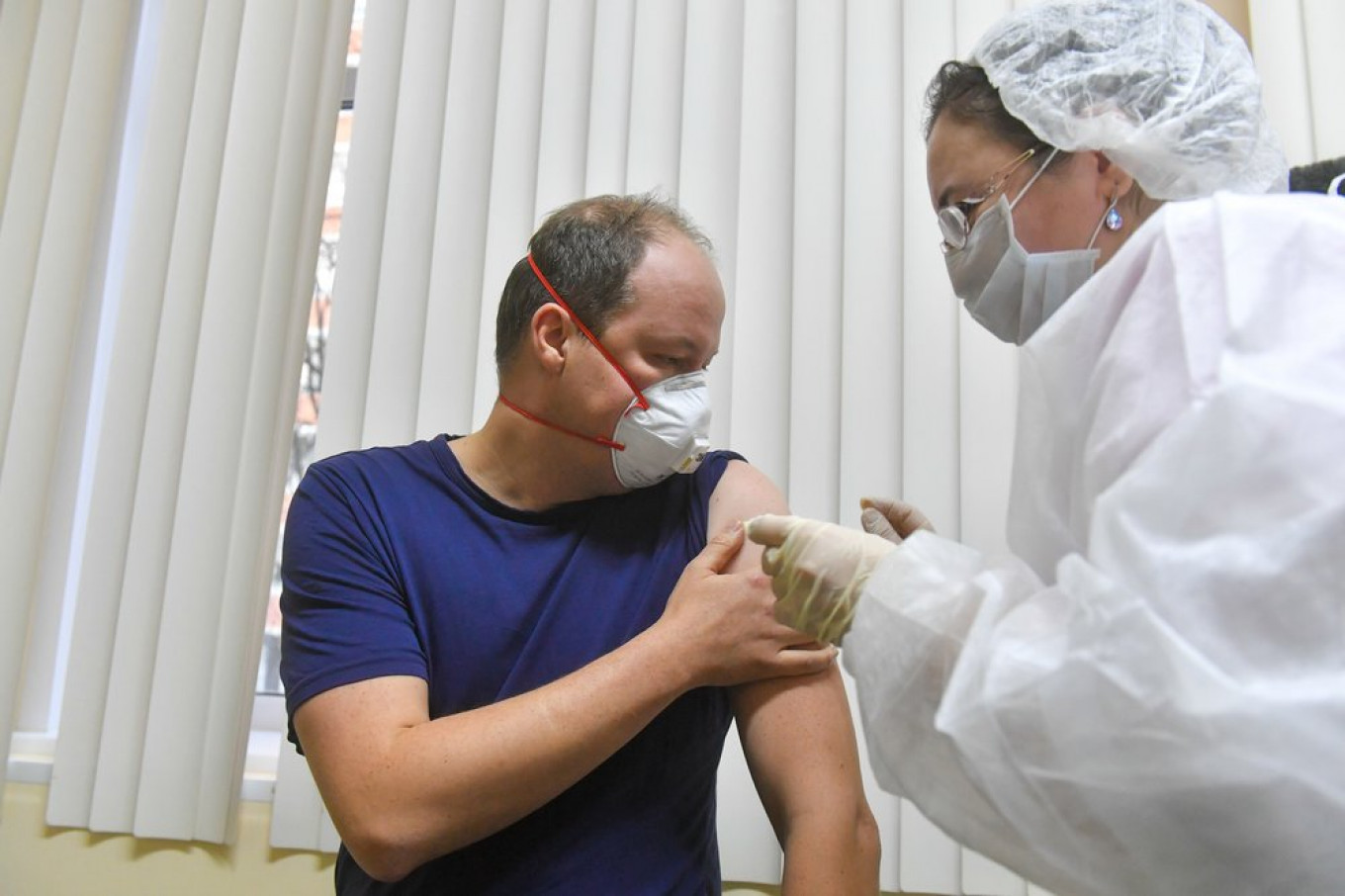
Russia launched its large-scale vaccination campaign starting with at-risk groups last week as ordered by President Vladimir Putin. The announcement came shortly after Britain issued the world’s first general-use approval to the Pfizer-BioNTech vaccine.
